Battery Materials And Their Components To Know
The whole world is running on electronics and as a result we are heavily dependent on one small product even is we do not seem to realise it and that is a battery. Nowadays even automotive vehicles are using improved batteries as a source of power. Solar energy can be made more usable and economical by storing it in batteries.
Three of the most used types of batteries are the Nickel-type Batteries, The Lithium Ion Batteries(Lib) and the Lead Acid Batteries all of which use different battery materials. The Lithium Ion Battery has become the most preferred form of battery owing to its high energy density which is a result of it's technology. There is a chance however, that Lithium Metal Batteries will even surpass Lithium Ion Batteries.
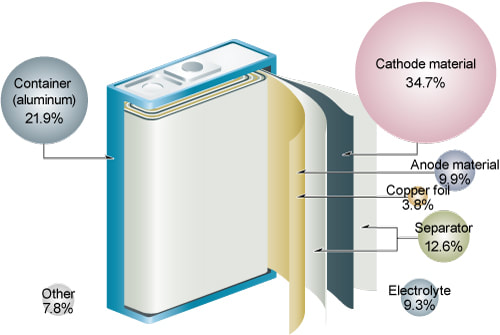
Most batteries nowadays are made up of four parts and the materials are - The Cathode, The Anode, Electrolyte and a Separator. Lithium Iron Phosphate, Lithium Manganese Oxide, Lithium Nickel Cobalt Manganese Oxide, etc are materials used for the Cathode. Anodes generally are made of graphite. Microporous polypropylene or polyethylene film is used for Separators while Electrolytes are generally LiPF6 or Lithium Hexafluorophosphate in Ethylene Carbonate or EC.
Raw Materials used in Batteries
The raw materials used in the construction of batteries are typically harvested from the Earth's Crust. In fact Living Beings, including Plants, also make use of these materials. However, each battery requires careful selection as to which battery materials are to be used in it and in what amount(s), so as to procure a harmonious interaction. Even a small mistake in the amounts can completely spoil the balance.
Here are a few of the raw materials -
Aluminium is a Silvery white, soft, nonmagnetic metal, with the symbol AI which on contact with air forms a layer which resists Corrosion on metal. On the other hand, Antimony is a brittle and lustrous metallic element used to reinforce lead plates in lead acid batteries and better their performances. It is also used for flame-proofing.
The soft gray alkaline metal that makes lead plates stronger and more efficient in lead acid batteries is Calcium. However, lead being soft, malleable and yet heavy it is the most obvious choice for lead acid batteries. Relatively soft in its purest form, iron can be hardened using carbon and finds use in lithium iron phosphate oxide batteries. Finally, an ion that is used in batteries is Chloride. It is negatively charged and forms on chlorine gaining an electron.
A few other raw materials that can be found in batteries are - Manganese, Nickel, Silver, Sodium, Spinel, Sulphur, Tantalum, Tin, Titanate, Vanadium, Zinc, etc.